From mouse to human: Cellular morphometric subtype learned from mouse mammary tumors provides prognostic value in human breast cancer
Breast cancer is the second deadliest cancer for women in the United States. How the malignant neoplasm develops, and the treatment necessary to combat the disease, is often as idiosyncratic as the individual affected.
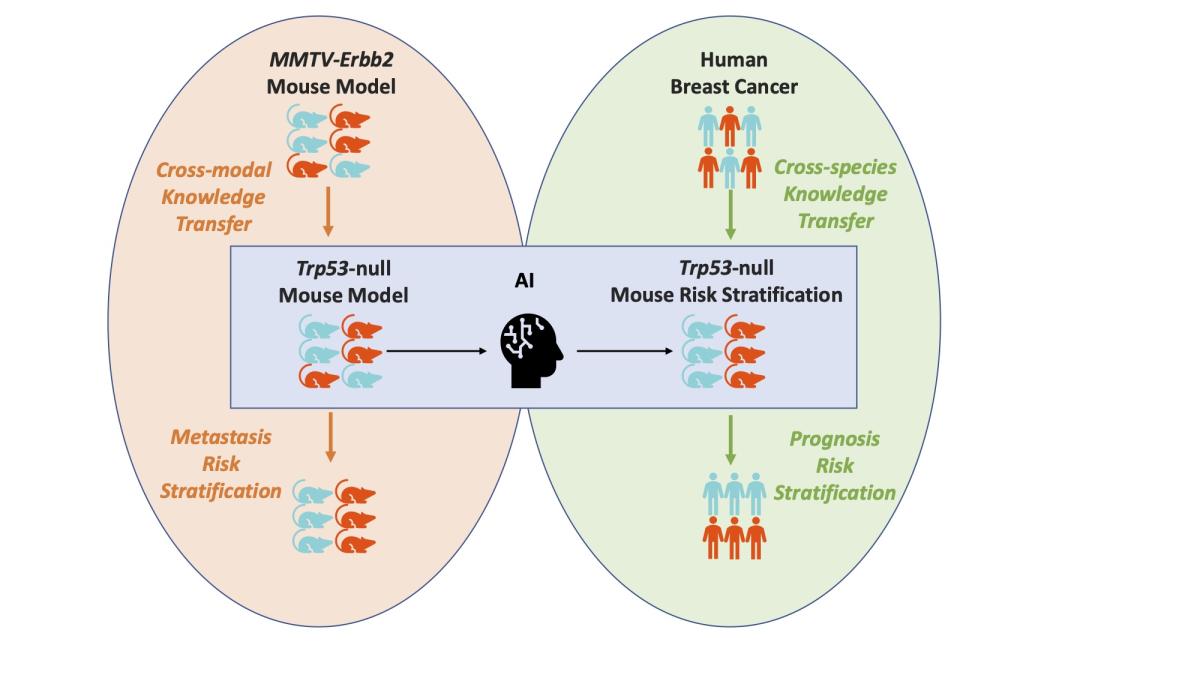
Scientists at Berkeley Lab, along with international collaborators, are working to expand our understanding of breast cancer diagnosis and treatment. Recently, a group in the Biological Systems and Engineering (BSE) Division developed a framework that enables the transfer of discoveries derived from mouse models to humans. This success will allow breast cancer researchers to better predict how likely a tumor in humans is to metastasize based on how the corresponding cells in mice behaved.
The team used microscopic tissue images of mouse tumors to discover cellular morphometric biomarkers (CMB), or cells that are likely to reveal a prognosis. Hang Chang, BSE research scientist and co-lead of the study, likened the function of CMBs to using genetic information to determine a gene that’s responsible for a given trait. Except in this case, CMBs help to predict the fate of a cancerous tumor.
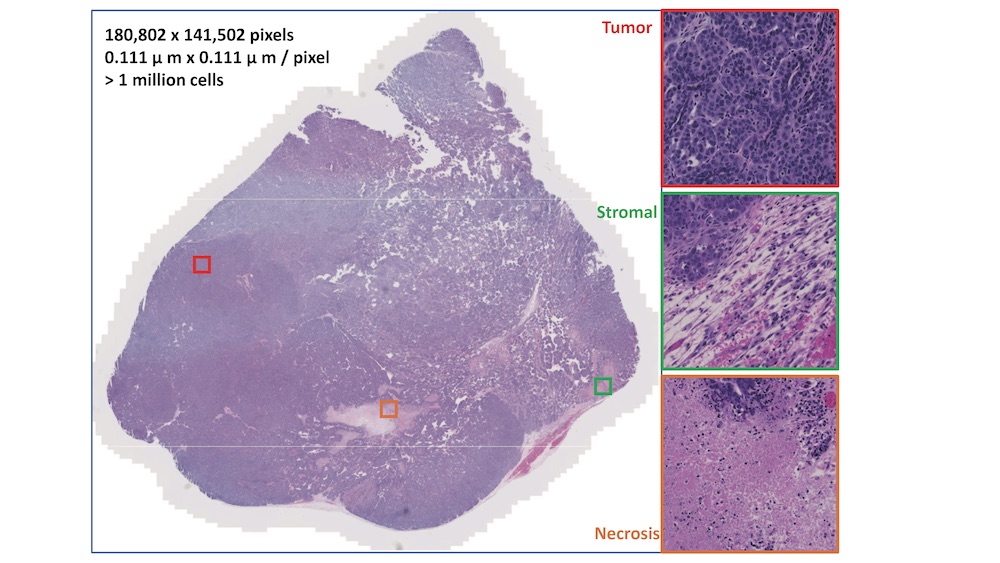
Representative example of a H&E stained mouse mammary tissue. (Photo credit: Hang Chang and Jian-Hua Mao)
“Due to the similarities of tumor microenvironments across different model systems, the CMBs discovered from the mouse model demonstrate promising capability for metastasis prediction in a different mouse model and risk for advanced disease in human patients,” Chang said.
Chang and project co-lead, BSE senior scientist Jian-Hua Mao, built a learning framework that transferred the mouse CMB information to a human model. They then used a publicly available data set from the National Cancer Institute (NCI) to validate the framework.
This machine learning pipeline is open source, meaning that anyone is able to access and use this transferable knowledge for their work. Beyond applications to breast cancer research, this framework could be applicable to any scenario when microscopic mouse cell information is plentiful but human data is scarce, including cross-species environmental risk screening and prevention.
Other BSE authors include: Xu Yang, Xiao-Ping Liu, and Antoine Snijders. Mary Helen Barcellos-Hoff at University of California San Francisco co-led the project; Kuang-Yu Jen at University of California Davis and Jesus Perez-Losada at University of Salamanca were contributors
This work was supported by the Department of Defense and the NCI of the National Institutes of Health.